February 2026 – For decades, Leiters Health has been a trusted, market-leading supplier of compounded and repackaged ophthalmic medications, supporting healthcare providers with the highest standards of quality, reliability, and service…
Pharmacy 503B Outsourcing refers to a specific type of pharmaceutical outsourcing facility regulated under Section 503B of the Federal Food, Drug, and Cosmetic Act (FDCA). It involves the outsourcing of compounded medications by a pharmacy facility that is registered with the U.S. Food and Drug Administration (FDA). These pharmacies are authorized to produce large quantities of compounded drugs for hospitals, clinics, and other healthcare providers, primarily for office use and without requiring individual prescriptions for each patient.
Pharmacy 503B outsourcing provides a crucial role in the healthcare system by producing bulk compounded medications that are safe, effective, and in compliance with FDA standards, ensuring that healthcare providers have the necessary medications to treat their patients in a timely and cost-effective manner.


Leiters is a trusted FDA-registered 503B outsourcing provider of high-quality ophthalmology and hospital-based services.
We are committed to providing healthcare professionals and their patients with high-quality medications.
Our team of experts in sterile pharmaceutical manufacturing, repackaging, and pharmacy provides a sophisticated understanding of what it takes to elevate the quality and consistency of supply in outsourcing.
We combine our team, our robust processes, and our state-of-the-art outsourcing facilities to ensure the highest quality products and services.
We believe the most important consumer of our products are patients, and patients have trusted Leiters with their health for nearly a century.
We are defining a new standard of quality that starts with an experienced, passionate team that cares about the health and safety of the patient. Our multi-disciplinary team of professionals consists of quality assurance experts, microbiologists chemists, and pharmacists. The experienced team ensures only the highest and most rigorous standards are satisfied for each batch of product manufactured, tested and released. The Leiters’ multi-disciplinary team brings years of experience from large sterile injectable pharmaceutical companies, hospital pharmacies, and academia. A quality mindset is deep rooted in our highly skilled team through extensive training, which includes cGMP training, and aseptic processing training. This highly trained team ensures quality, consistency and compliance with all released products. Leiters stands apart by focusing on one singular purpose: bringing health to outsourcing.
This is the place where quality means everything.
Our FDA-registered 503B outsourcing facility is located in Denver, Colorado. With increasing regulatory pressure, there is a growing need for higher standards in pharmaceutical outsourcing. Leiters’ facility in Denver was designed to exceed traditional outsourcing facilities and was constructed with the patient in mind. We are defining a source for greater accountability with our facility in Colorado. Our state-of-the-art facility ensures quality, consistency and compliance with all released products.
We invite you to visit our facility to better understand the cGMP regulations, sterile manufacturing processes, and automation we use to elevate the quality of our products and services. Come join the growing list of organizations who have visited our facilities.
Defining a source for greater accountability is imperative to drive better medicine. We believe that making better medicine starts with a broader perspective. Prioritizing what matters most and putting patients at the center of everything that we do helps us make better medicine. All sterile hospital and ophthalmology preparations are produced under the Human Drug Outsourcing Facilities under 503B of the FD&C Act (503B Guidance), Current Good Manufacturing Practices (cGMP) and exceed USP <797>. This strict set of manufacturing standards is designed to ensure the highest quality of compounded medications and maximum patient safety. For a full list of available hospital and ophthalmology products, see Products tab. Leiters compliance with strict guidance ensures quality, consistency, and compliance with all released products. Leiters stands apart by focusing on one singular purpose: bringing health to outsourcing.
The Pharmacy500 is awarded to 500 pharmacy supply chain businesses and associations that significantly impacted dispensing pharmacies in the U.S. over the past year.
The following are complimentary benefits extended to Pharmacy500 companies and associations (coming this spring!)


At CenPharma, we empower 503B pharmacies by ensuring they meet the highest standards of GMP compliance. Our team of experienced consultants provides tailored solutions for pharmaceutical companies of all sizes, focusing on compliance, quality management, and operational efficiency.
In an environment of constant regulatory change, having an experienced partner is crucial. CenPharma understands the unique operational and quality needs of 503B pharmacies, delivering customized consulting that meets you where you are. We’ll work alongside you to assess your current practices, identify areas for improvement, and develop a practical roadmap to achieve and sustain compliance.
Let’s navigate the complexities of GMP compliance together.
Visit us at Booth #13848 to learn how we can support your journey towards operational excellence and regulatory assurance.
Join us to build a future of reliability and success in pharmaceutical compounding.


















February 2026 – For decades, Leiters Health has been a trusted, market-leading supplier of compounded and repackaged ophthalmic medications, supporting healthcare providers with the highest standards of quality, reliability, and service…
February 2026 – Leiters Health is pleased to announce the addition of a new presentation of Lidocaine pre-filled syringes to our growing portfolio. This expansion reflects our ongoing commitment to listening to our customers and responding with solutions that support more efficient care delivery…
February 2026 – Leiters Health is pleased to announce the addition of a new presentation of Phenylephrine pre-filled syringes to our growing portfolio. This expansion reflects our ongoing commitment to listening to our customers and responding with solutions that support more efficient care delivery…
Leiters Health supports hospital pharmacies by offering 503B services for ready-to-use sterile compounded medications including prefilled syringes, IV bags, ophthalmic products, pain management solutions, and repackaged Avastin. To learn more, visit the 20Ways Winter Hospital publication.
This 20Ways profile highlights Verity solutions and how their cloud-based solutions streamline 340B management, purchase optimization, real-time pricing, contract price validation, and infusion management. To learn more, visit the 20Ways Winter Hospital publication.
In this case study, we take a look at Leiters Health partnered with Mobile Infirmary to pilot ready-to-dilute concentrated norepinephrine vials, significantly reducing preparation and administration time and freeing up IV-room resources.
In this case study, learn how Charles River Laboratories enabled JSD Pharmacy to surpass standard 503A compounding criteria, employing rapid endotoxin detection, advanced microbial identification, and robust data tools to deliver heightened quality control and patient-safety assurance.
In this case study, see how Charles River Laboratories helped Fagron Sterile Services strengthen quality control by combining Endosafe rapid endotoxin testing and Accugenix microbial identification, delivering faster results, regulatory confidence, and unmatched reliability for 503B compounding operations.
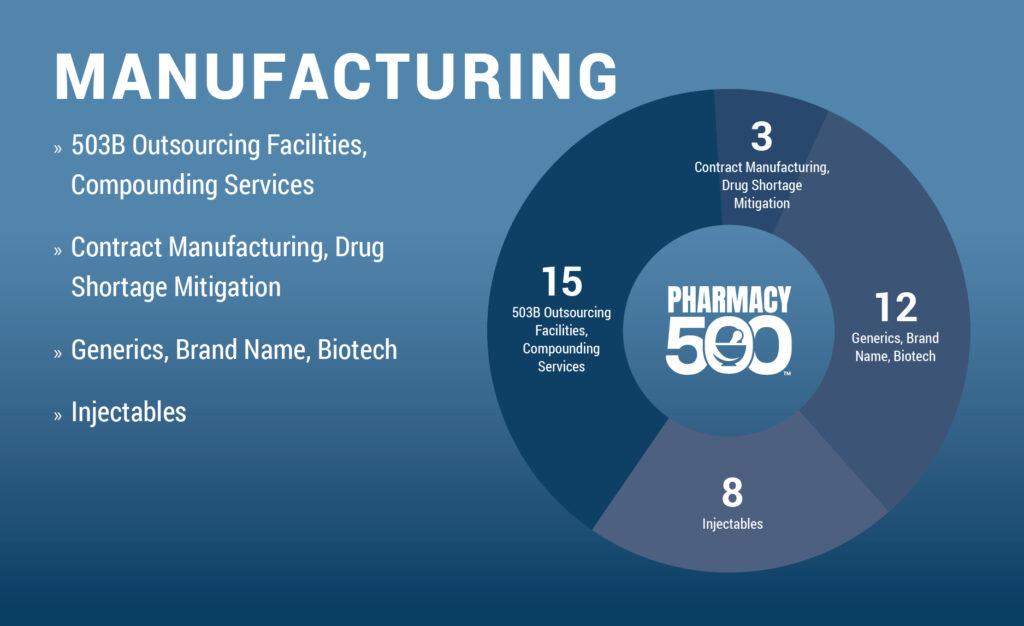
This report aims to educate Pharmacy500 professionals, pharmacy leaders, and industry stakeholders on the 503B compounding sector and its critical role in healthcare. 503B outsourcing facilities, regulated by the FDA, produce large quantities of sterile and non-sterile compounded medications to help address drug shortages and support clinical needs. By ensuring safety, compliance, and reliability, these facilities strengthen medication access and continuity of care across the healthcare system.
RXinsider’s Pharmacy500 Academy now features comprehensive training on FDA 503B outsourcing facilities and insurance-supported 503B services, including detailed product comparisons and company profiles. Visit the Pharmacy500 Academy to explore and learn more about 503B outsourcing solutions.
August 2025 – Ketamine was recently removed from the FDA Drug Shortage List. In alignment with our commitment to the highest standards of regulatory compliance, Leiters Health, an FDA-Registered 503B provider, has transitioned our production of Ketamine syringes to source exclusively from finished dose product (FDP), rather than active pharmaceutical ingredient (API).
AIS Healthcare
Amneal Pharmaceuticals LLC
Azurity Pharmaceuticals, Inc.
B. Braun Medical Inc.
Baxter
Beutlich Pharmaceuticals LLC
Central Admixture Pharmacy Services, Inc. (CAPS)
Cipla, Inc.
Civica
Dr.Reddy’s Laboratories Ltd.
Endo USA
Fagron
Fresenius Kabi Group
Germfree
Grifols
Hikma Pharmaceuticals
Leiters Health
Lupin Pharmaceuticals Inc.
Medivant Healthcare
Moderna, Inc.
Nephron Pharmaceuticals Corporation
OurPharma LLC
Pine Pharmaceuticals
Precision Dose, Inc.
QuVa Pharma
Revelation Pharma
SCA Pharma
STAQ Pharma, Inc.
Turbare Manufacturing, LLC
Wells Pharma
West Pharmaceutical Services, Inc.