Why USP<797>?
- Earlier efforts were unsuccessful since USP 1206 was voluntary and initial USP<797> was confusing and not comprehensive
- Reduce or prevent harm to patients resulting from bioburden, endotoxins and other contamination of compounded sterile products
- The guidance represents a process to gain a documented state of control over pharmacy operation and performance
- Final revision in November of 2023 is now the standard of practice
Why USP<800>?
- Implement a Quality System that creates a documented state of control over the pharmacy
- Outline containment strategies for Hazardous Drugs HDs
- Facility and Engineering Controls
- Protective Equipment
- Work Practices
- Promote worker and patient safety through protection from exposure to HDs
- Provide a framework to assess the risk of HDs
USP <797> <800> November 2023
- Continued emphasis on Pharmacy Management Responsibility for Quality System with defined components
- Addresses deficiencies, contradictions and ambiguities
- Invest in a Quality Assurance System now or pay the “penalty” later approach
- Microbial and Chemo Wipe Testing/Trending to demonstrate control over the pharmacy cleaning, material and personnel work flow
- State Boards, JCAHO and others have adopted as the standard of practice and are inspecting to the standard
Management Strategies
- Document your plan
- Define the resources needed to implement your plan
- Develop a process to investigate excursions and maintain the pharmacy in a documented state of control
- Focus on changing staff compounding culture
- Full Commitment from Management and Personnel
- Utilize specialized providers that can manage and report testing data as a tool to implement Quality System requirements
Quality Assurance Program
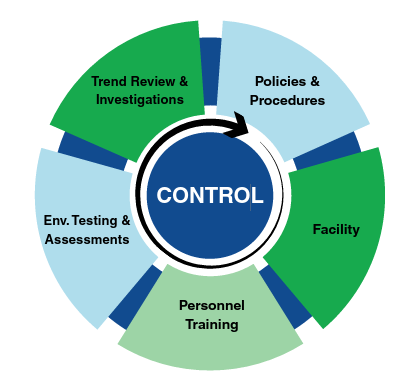
How Quality Solutions Can Help
- Certification & Environmental Testing
- Environmental Monitoring (EM) Kits
- Facility Design and Assessment
- Personnel Training & Assessment Support
- Quality System and Compliance Consulting
EM Kits
- Provide Kits and Reports to self perform routine environmental sampling
- Support your in-house testing with incubation and enumeration of test samples
Ensuring a Return on the Cost of Quality
800.935.1146
www.qualsolve.net
info@qualsolve.net





