
Amneal Pharmaceuticals, Inc. (NYSE: AMRX) is an integrated specialty pharmaceutical company powered by a robust U.S. generics business and a growing branded business. Together, our team is working to build one of the most dynamic pharmaceutical companies in our rapidly changing industry.






























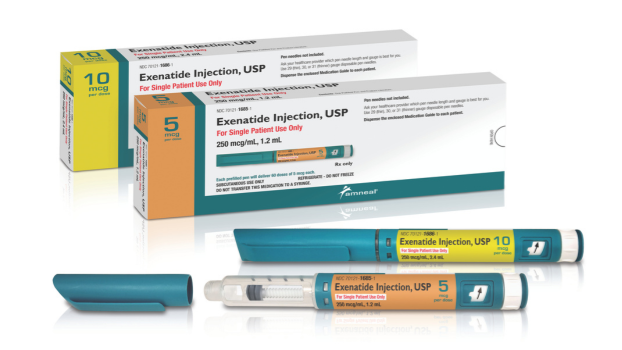



















![Ready-to-Use Products from Amneal Allow You to Focus on What Matters Most | Amneal [VIDEO]](https://internal.rxinsider.com/file/amneal-a567-1732305136.webp)

















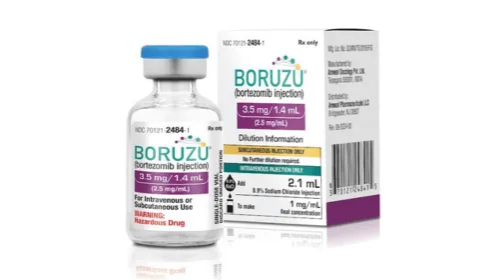
![Amneal Pharmaceuticals | We’re Branching Out Into Premix Bags [Video]](https://internal.rxinsider.com/file/amneal-8139-1728270868.webp)





































