Contributed by: Clinical Program Managers, Annie Lambert, PharmD, BCSCP,
and Jennifer Smith, PharmD, BCSCP, at Simplifi+ Pharmacy Compliance Solutions, Wolters Kluwer
Hazardous Drugs (HDs) are present throughout the healthcare setting, posing risks to the personnel that handle them. While many drugs have therapeutic effects for patients, acute or long-term exposure to staff can cause adverse effects such as nausea or respiratory irritation, reproductive challenges, and even cancer.
The United States Pharmacopeia (USP) published General Chapter <800> Hazardous Drugs — Handling in Healthcare Settings initially in 2016 to set standards for the protection of healthcare workers and the environment. Even with years of evidence and guidance documents, practices to reduce the risk of HD exposure were not widely adopted until the creation of USP <800>. Enforcement was further delayed until USP <800> became compendially applicable as of November 1, 2023i , when it was referenced in both USP <797> Pharmaceutical Compounding – Sterile Preparations and USP <795> Pharmaceutical Compounding – Nonsterile Preparations.
USP <800> applies to all facilities and personnel that handle, prepare, or administer HDs, including hospitals, clinics, pharmacies, long-term care facilities, infusion centers, veterinary offices, and other healthcare settings where HDs are handled. The primary goal of USP <800> is to minimize the risk of HD exposure to the environment, patients, and healthcare workers handling these drugs throughout the facility. This includes receiving, storing, compounding, transporting, administration, disposal, and spill cleanup. Following safe handling procedures outlined in USP <800> reduces the risk of HD exposure via prevention and containment.
With all the delays, you may need a refresher on the importance of safe handling of hazardous drugs. This article will provide an overview of USP <800> requirements, considerations for implementation, and future directions for compliance.
Key Components of USP <800>
HAZARDOUS DRUG LIST: A facility-specific list of HDs must be generated to understand and identify HDs at the facility. HDs are defined by USP <800> based on the criteria established by NIOSH. NIOSH maintains a list of HDs with the most recently published document from 2016, the NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016, also called the NIOSH List. The criteria for a drug to be defined as hazardous and included on the NIOSH List include drugs that are toxic to organs at low doses, carcinogenic, teratogenic, reproductive, or genotoxicii. NIOSH groups HDs into three categories: Table 1, 2, or 3.
Table 1 drugs are antineoplastic drugs that pose significant health risks from exposure.
Table 2 drugs are non-antineoplastic drugs that meet one or more NIOSH HD criteria and pose a potential risk from exposure.
Table 3 drugs meet the NIOSH criteria for reproductive hazards to men or women trying to conceive and women who are breastfeeding.
All Table 1 drugs on the NIOSH List and all HD Active Pharmaceutical Ingredients (APIs) must be handled with all the containment strategies and work practices required by USP <800>. However, the Chapter allows organizations to develop an Assessment of Risk (AOR) for Table 2 and 3 drugs and final dosage forms of compounded HD preparations and HD (including antineoplastic) dosage forms not requiring further manipulation. An AOR approach determines if alternate containment strategies or work practices may be employed to limit HD exposure instead of the full protective measures described in the Chapter.
FACILITY DESIGN: The requirements for HD facility design focus on containment. This is likely the most challenging component of USP <800> for facilities to achieve since re-design and construction may be required to bring existing facilities into compliance. With the elimination of the “low-volume” HD exemption found in the 2008 version of USP <797>, all HD compounding must occur in a containment secondary engineering control (C-SEC) maintaining negative pressure and external ventilation. Additionally, HDs on NIOSH Table 1 or API of any HD must be stored in a negative pressure environment. When a separate HD storage room is not attainable at the facility, HDs can be stored in C-SECs provided storage does not negatively affect engineering controls or increase the microbiological burden in sterile compounding rooms. The following table summarizes the minimum facility requirements for HD compounding and storage areas.
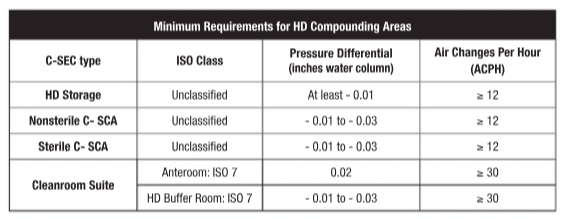
Containment primary engineering controls (C-PECs) for sterile HD compounding require external ventilation and negative pressure. In contrast, C-PECs for nonsterile HD compounding can be externally vented (preferred method) or have redundant HEPA filters in a series.
PERSONAL PROTECTIVE EQUIPMENT: Ensuring staff compliance with Personal protective equipment (PPE) serves as the last level of control against HDs from exposure. PPE worn when handling HDs must be outlined in standard operating procedures (SOPs) and is based on the activity personnel are performing including all or some of the following PPE:
• ASTM standard D6978iii gloves (single or double and sterile or nonsterile based on activity).
• Long-sleeved impermeable gowns that close in the back and have elastic cuffs.
• Shoe covers (double shoe covers required during HD compounding).
• Head and facial hair covers.
• Face and eye protection.
• Respiratory protection.
Removal of HD PPE and hand hygiene is equally important to prevent cross-contamination after the completion of HD handling activities.
TRAINING & COMPETENCY: All personnel who handle HDs must receive training and demonstrate competency. HD training needs to supplement general education and competency for handling any drugs and occurs in addition to USP <797> and <795> training. When considering the components of an HD training program, all personnel handling HDs must receive training on identifying HDs, organizational HD SOPs, PPE use, correct use of engineering controls and other devices, response to HD exposure, HD spill management, and proper HD disposal.
DEACTIVATION & DECONTAMINATION: USP <800> introduces the requirement for deactivation and decontamination before cleaning and disinfection of areas where HDs are handled. Deactivation renders the HD inactive, while decontamination removes the residue by transferring it to a disposable material like a low-lint wiper. Deactivation and decontamination occur in addition to the cleaning and disinfection, or sanitation required by USP <797> and USP <795>. Ensure personnel training and organizational SOPs outline PPE worn along with the agents and supplies for each deactivation, decontamination, and cleaning step.
Implementing USP <800>
GAP ANALYSIS: The first step to the implementation of USP <800> standards is to assess current practices. Since the standards have been available since 2016, many pharmacies may have started to implement elements of the Chapter but paused progress when enforcement was delayed. Several gap analysis tools are available online such as, readyfor800.com or from state boards of pharmacy.
Also key to the assessment of current practices is knowing what HDs are handled in your facility and where. Begin by reviewing purchasing records or dispensing logs and compare them to the NIOSH List to identify what HDs have been procured. Then follow the HD through the process from receiving through dispensing and administration, even to waste and disposal. This exercise may identify additional gaps or personnel that require training. Understanding what HDs are present will lead to further discussion about how they should be handled to ensure personnel safety.
DESIGNATED PERSON: In addition to conducting a gap analysis, it is also important to identify who will be the point person for USP <800> compliance. The designated person is responsible for oversight of all aspects of the hazardous drug safety program and should have the authority to implement change when needed. This may be a committee or multiple individuals, just be clear to describe the roles and responsibilities to ensure all aspects are assigned.
POLICIES & PROCEDURES: Foundational to any compliance program are policies and procedures outlining how standards will be adopted at the facility level. USP <800> requires entities to maintain SOPs to include at least 16 different topics including receipt and storage to hand hygiene and use of PPE to transport and spill control. SOPs must be reviewed at least every 12 months by the designated person. Developing SOPs can be daunting at first, but maintenance should be minimal once the standards are established.
FACILITY UPDATES: For HD compounding and storage activities, facility modifications and upgrades may be required to achieve the required negative pressure, external exhaust, and air exchanges. Additional primary engineering controls may also need to be purchased and vented to the outside. Engage with your facilities and engineering team to understand the current HVAC design and capabilities. Ensure these teams realize the minimum USP <800> requirements and collaborate to discern how these align with other environment of care standards, such as those from ASHRAEiv and OSHA.
TRAINING & COMPETENCY: After knowing what HDs are handled in your facility, understanding the types of exposure, and establishing SOPs for the containment of HDs, personnel must receive training based on their job functions. This includes general education such as the risks of handling HDs and proper use of PPE and engineering controls, as well as facility specific training related to the entity’s list of HDs, SOPs, and response to and HD exposure and spill management. Developing an HD training checklist ensures all personnel complete all required HD training initially and annually during employment.
ANNUAL REVIEW: All the aspects described above must be reviewed or repeated at least every 12 months to ensure new information is incorporated and safe handling practices are maintained. Engaging stakeholders in this process is encouraged as front-line personnel are ultimately the ones at greatest risk during their day-to-day responsibilities.
Future Trends & Developments
Even with the delays in enforcement of USP <800>, many entities have moved forward with compliance and other aspects of HD safety have continued to evolve. Here are a few topics to have on the radar:
• NIOSH Updates: The current official NIOSH List of Hazardous Drugs in Healthcare Settings was published in 2016. Several drafts have been proposed, but no new updates have been finalized. When the next revision is published, it will include a change in classification of HDs, going from three groups to two groups which may require entities to revise SOPs and approach to HD identification and precautions.
• ASTM Gown Standard: In late 2022, the American Society for Testing and Materials established a testing standard for chemotherapy gowns. ASTM F3267-22v establishes design and performance standards for protection against chemotherapy and other liquid HDs. Similar to ASTM D6978 for gloves, this will help ensure PPE protects workers based on evidence. Look for this testing standard when evaluating PPE.
• Compounding Automation: Technology-assisted workflow solutions continue to evolve to meet the needs of various compounding settings. For facilities that compound a higher volume of HDs, implementing compounding robotics should be considered as a method to increase safety and decrease exposure to HDs.
• HD Wipe Sampling: Testing for HD residue is currently a recommendation in USP <800>, though many organizations have already implemented this practice. HD wipe sampling can be used as a routine component of a quality assurance/quality control program and/or to verify effective clean-up of an HD spill. Several testing kits and vendors are available, providing both qualitative and quantitative results.
• Medical Surveillance: Monitoring of workers exposed to HDs for health conditions is also a best practice recommendation in USP <800>. Implementation of a medical surveillance program requires a coordinated effort with employee health, human resources, and legal and risk teams. Refer to guidance in USP <800> and from OSHAvi.
Conclusion
With nearly a decade since USP <800> was published, the standards may still feel new and overwhelming. The Chapter provides many of the expectations for what is required to protect healthcare workers and the environment. Many of the nuances of how to implement the standards exist in facility SOPs. Organizations further along in the compliance journey may now be encountering annual reviews and adjusting their initial safe handling practices based on feedback from staff and experience with HDs. As enforcement of the standards moves forward, additional technologies and resources will emerge to further reduce the risk of HD contamination.
Ultimately, the USP <800> standards exist to protect healthcare workers and the environment, and reduce exposure to HDs overall. The risks are clear, and the evidence is abundant. Regardless of the practice setting, implement appropriate precautions and strive for consistent compliance with the standards.





