What is stability?
The extent to which a product or preparation retains physical and chemical properties and characteristics within specified limits throughout its expiration or beyond use date (BUD).
When is stability data required?
Compounders must consider the chemical and physical stability of the compounded preparation and its components when establishing a BUD. Stability data utilizing a stability-indicating analytical method is required to:
· Determine if a shorter BUD must be assigned when the stability is less than the limits specified in USP 795 and 797
· Extend BUD for nonsterile preparations up to a maximum of 180 days
· Support BUD for Category 3 sterile preparations
What tests are included in a stability study?
Tests are based on dosage form, formulation, and product properties and demonstrate chemical, physical, and microbiological stability. Tests may include:
· Sterility (for sterile preparations)
· Endotoxin (for most sterile preparations)
· pH
· Appearance
· Particulate matter
· Potency/ Assay
· Antimicrobial effectiveness testing for aqueous preparations
· Preservative quantification
· Microbiological tests for water-containing formulations
· Microbial Enumeration Tests (for nonsterile preparations)
· Tests for Specified Microorganisms (for nonsterile preparations)
· Container closure integrity
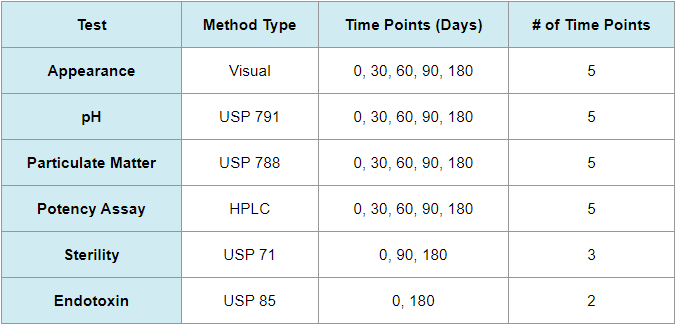
How many time points are included in a stability study?
The timing for conducting tests is determined based on the desired beyond-use date. ARL recommends conducting tests at the beginning, end, and at least three time points. Not all tests need to be conducted at every time point, but doing so allows for a more comprehensive analysis of the preparation’s stability and provides intermediate time points to use if a test result is out of specification. The stability design may vary depending on the test type and the frequency required by USP guidelines.
Stability Study Design Example:
How long does a stability study take?
The stability study turnaround time depends on the stability-indicating analytical method. For a method to be considered stability-indicating, the method must be developed and validated per USP 1225. The validation includes accuracy, linearity, precision (repeatability), range, specificity, and system suitability. Specificity demonstrates non-interference from impurities and matrix components and involves stress studies/ forced degradation to demonstrate the method is capable of separating degradation products from the active pharmaceutical ingredient (API).
Stability Indicating Analytical Method Options:
Method Development and Validation:
ARL develops and validates a stability-indicating analytical method for the quantitation of the API(s). After the method is validated, ARL evaluates the physical, chemical, and microbiological properties of the packaged product over a target expiration period.
Method development and validation must be performed on the exact product being tested to eliminate interference from degradants and impurities.
Development Time: 8 – 12 weeks (time varies based on formulation complexity)
Method Verification:
ARL verifies a previously validated stability-indicating analytical method is appropriate for the quantitation of the API(s). The laboratory demonstrates specificity for matrix components, system suitability, and accuracy of the previously validated method. After the method is verified for the formulation, ARL evaluates the physical, chemical, and microbiological properties of the packaged product over a target expiration period.
Method verifications can be used on the same or similar products tested with fewer APIs and/or excipients.
Verification Time: 4 – 6 weeks
Method Applicability Assessment:
ARL compares the drug product being tested to a previously validated stability-indicating analytical method and performs system suitability analysis. A successful system suitability analysis indicates no difference between the product being tested and the previously validated formulation.
Applicability Time: 4 weeks (time varies based on tests performed and GMP vs. non-GMP)
When are stability protocols required?
Protocols for method work and stability are required for GMP stability-indicating analytical methods. The protocol directing the method work will specify the methods and acceptance criteria used to validate the method. The stability study protocol will specify the tests, methods, timepoints, and acceptance criteria applied during the stability study.
What is ARL’s wait time to start method development, verification, or assessment?
ARL’s wait time fluctuates with the seasons and demand. To avoid delays, we highly recommend submitting a quote request ahead of time and preparing for a minimum 12-week wait.
Currently, there is no wait time for ARL to start work on your method.
Contact ARL for more information on stability studies info@arlok.com or 800-393-1595.