Contributor: Alex Atmadi, MSC., Scientific BU and Product Magner, ESCO Technologies, Inc.
Q. What are the key differences between pharmacy isolators, cleanrooms, and barrier isolators in terms of functionality and regulatory compliance?
Pharmacy isolators, cleanrooms, and barrier isolators (also known as Restricted Access Barrier Systems or RABS) serve distinct roles in pharmacy operations, each with unique functionalities and regulatory compliance requirements. Both pharmacy isolators and RABS are categorized as Primary Engineering Control (PEC) while cleanrooms can be categorized as Secondary Engineering Control (SEC) where the PEC is housed.
• Pharmacy isolators are designed to provide a controlled environment for aseptic processing and compounding of sterile drugs. They are fully enclosed systems that prevent contamination from external sources. Pharmacy isolators need to be pressure tested according to ISO 14644-7 and ISO 10648-2 with a stringent leak rate. Thanks to this design principle, pharmacy isolators can be equipped with a quantifiable and automated decontamination system, as well as good reliability for toxic containment.
• Barrier isolators, or RABS, also provide a controlled environment and an enclosure with the separative partial barrier, however, as per CETA CAG-002-2006, there is no requirement for the RABS to be pressure tested. RABS will be highly dependent on operator aseptic techniques to reduce the contamination risk and has low capability in toxic containment.
• Cleanrooms are also designed to provide a controlled environment via an open system and will refer to regulatory standards like ISO 14644.
In summary, while pharmacy isolators, cleanrooms, and barrier isolators all aim to ensure safe and effective compounding processes, they differ significantly in functionality and regulatory compliance requirements.
Q. How would a facility determine the appropriate cleanroom classification needed for their specific pharmacy operations?
First, the pharmacy facility will need to define the compounding activities and risk levels. Will it be for non-sterile compounding or sterile compounding? If it is sterile compounding, will it be a sterile hazardous (HD) or non-hazardous (non-HD) compounding? Will the pharmacy operations produce Compounded Sterile Preparations (CSP) Category 1 or Category 2?
The U.S. Pharmacopeia (USP) standards, especially USP <797> and USP <800>, outline cleanroom requirements for sterile compounding (797) and hazardous drug handling (800). Critical areas where sterile compounding occurs, whether it is hazardous or non-hazardous drugs, should maintain an ISO Class 5 classification. This air cleanliness can be achieved with the use of Primary Engineering Control (PEC), which can be either a pharmacy isolator or RABS.
Depending on the product categories based on the risk of microbial contamination and whether the CSPs are HD or non-HD, the pharmacy facility can consider the following:
• For non-HD CSPs with a beyond-use date of less than 12 hours stored in temperature or less than 24 hours refrigerated, PECs (laminar flow hoods, biosafety cabinet class II, and compounding aseptic isolator/RABS) can be located in an unclassified room or segregated compounding area with no air changes per hour requirement.
• For non-HD CSPs with a beyond-use date of less than 24 hours stored in temperature or more than 24 hours refrigerated, PECs (laminar flow hoods, biosafety cabinet class II, and compounding aseptic isolator/RABS) must be located in a positively pressured ISO Class 7 cleanroom as buffer room with ISO Class 8 cleanroom as ante-room. The ISO Class 7 cleanroom must achieve more than 30 air changes per hour. In special cases with the use of a pharmacy isolator, this PEC can be housed in an ISO Class 8 cleanroom as a buffer room with fewer air changes per hour requirement of 20.
• For HD CSPs with a beyond-use date of less than 12 hours stored in temperature or less than 24 hours refrigerated, PECs (biosafety cabinet class II, and compounding aseptic isolator/RABS) can be located in an externally vented, negatively pressured unclassified room or segregated compounding area with minimum 12 air changes per hour.
• For HD CSPs with a beyond-use date of less than 24 hours stored in temperature or more than 24 hours refrigerated, PECs (biosafety cabinet class II, and compounding aseptic isolator/RABS) must be located in an externally vented, negatively pressured ISO Class 7 cleanroom as buffer room and ISO Class 7 ante-room with minimum 30 air changes per hour.
Due to better sealing, in non-hazardous CSP settings, pharmacy isolators can produce preparations with longer beyond-use dates even when they are placed in less strict cleanrooms.
Q. Can you provide insights into the best practices for maintaining and validating cleanroom environments, including the role of cleaning supplies like wipes and mops?
Maintaining and validating cleanroom environments requires rigorous, routine cleaning protocols and specialized supplies to control contamination effectively.
• Daily and shift-based cleaning using lint-free wipes, disposable, non-shedding mops, and pre-saturated disinfectant wipes help ensure consistent disinfection, with rotation between different disinfectants to prevent microbial resistance. A thorough cleaning should consider high-touch surfaces such as work benches, PECs, and door handles. The frequency of this high-risk equipment should be cleaned at least once per shift or daily.
• Environmental monitoring, including particle counting and microbial sampling, verifies air and surface cleanliness against ISO standards.
• Top-to-Bottom, Back-to-Front Technique Implementation where the cleaning starts from the cleanest areas (top) to the least clean (bottom), from the back to front to prevent re-contamination of already-cleaned surfaces.
• Proper entry and exit protocols, including gowning and equipment sterilization, reinforce contamination control.
• The validation of HVAC and HEPA systems, air changes per hour validation, and cleanroom pressure maintenance are critical to meeting cleanroom requirements.
• Clear SOPs and comprehensive documentation of cleaning activities, coupled with regular personnel training and certification, uphold regulatory compliance and ensure the reliability of cleanroom operations.
Q. What are the essential requirements for USP-compliant supplies, and how a facility ensure the facility meets these standards?
Essential requirements for USP-compliant supplies are sterile, non-shedding, and chemically compatible supplies, like lint-free wipes and sterile alcohol, to maintain a classified environment. Facilities should select USP-compliant materials that support the ISO classification of the cleanroom and implement a robust environmental monitoring program to regularly test air, surfaces, and particle levels to meet USP <797> or <800> standards. The facility ensures compliance by establishing comprehensive SOPs for cleaning frequency, procedures, and agent selection, as well as providing staff training in aseptic techniques and owning.
Q. How does the integration of advanced filtration systems impact the overall performance and safety of pharmacy isolators and cleanrooms?
The integration of advanced filtration systems significantly enhances the performance and safety of pharmacy isolators and cleanrooms by improving air quality and reducing contamination risks. High-efficiency particulate air (HEPA) and ultra-low particulate air (ULPA) filters capture up to 99.9995% of particles at 0.3 microns, including bacteria and other contaminants. The use of an advanced filtration system ensures the cleanroom environment meets stringent ISO classifications and USP standards for sterile compounding.
Advanced filtration also allows for more effective removal of hazardous materials, including cytotoxic particles, which is critical in handling hazardous drugs in compliance with USP <800> guidelines. The use of this filtration system in a pharmacy isolator’s exhaust system will also impact both operator and environmental safety. By maintaining stable airflow patterns and filtration efficiency, these systems help ensure aseptic conditions, reduce the risk of product contamination, and protect both staff and patients from exposure to harmful substances. Regular monitoring, validation, and maintenance of these filters are essential to uphold safety and operational standards, ultimately enhancing the reliability and safety of pharmacy operations.
Q. What training or certifications are recommended for pharmacy staff to effectively use and maintain clean air technology systems?
Pharmacy staff should receive specialized training and certifications to effectively use and maintain clean air technology systems. Recommended areas include aseptic technique training, USP <797>, and <800> compliance for handling sterile and hazardous drugs. Together, these credentials ensure staff can uphold high standards of cleanliness, safety, and regulatory compliance in pharmacy operations.
Q. What are the cost implications and potential return on investment (ROI) when upgrading to the latest cleanroom technology and fixtures?
Upgrading to the latest cleanroom technology and fixtures involves significant initial costs, including the purchase of advanced equipment, installation, and ongoing maintenance. Training staff on new systems also adds to the investment. However, the potential return on investment (ROI) can be substantial. In general, an ISO Class 8 cleanroom with a pressure-tested pharmacy isolator is typically more cost-effective than an ISO Class 7 cleanroom with RABS.
For example, given the case of establishing a new facility for non-HD CSPs.
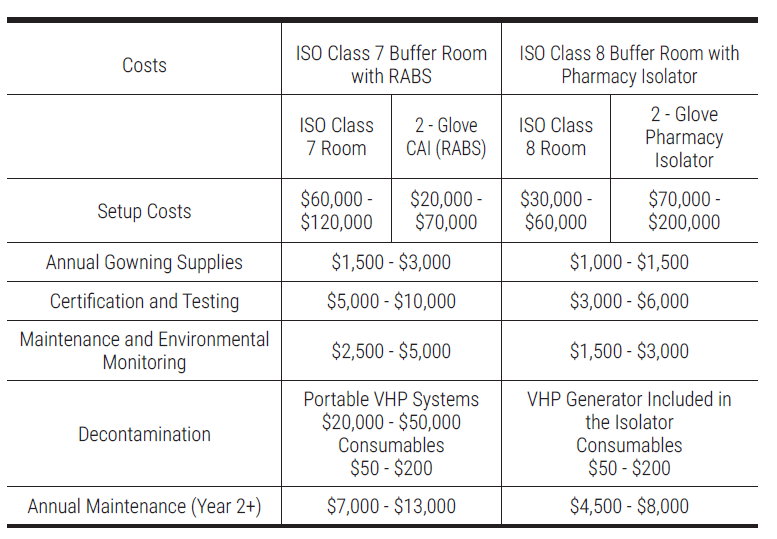
Improved compliance with regulations enhances patient safety and reduces the risk of costly penalties, while modern systems increase operational efficiency and throughput. Upgraded filtration and containment technologies can minimize product waste, leading to higher yields and lower disposal costs. Additionally, energy-efficient equipment can result in long-term savings on utility and maintenance expenses.
Ultimately, while the upfront costs may be high, the long-term benefits such as enhanced competitiveness, improved reliability, and increased profitability make a compelling case for investing in cleanroom and PEC upgrades.








