Sterility and Endotoxin
Kayla Lipcaman, ARL Bio Pharma Associate Microbiology Supervisor
Two critical tests for sterile compounded preparations are Sterility and Endotoxin testing.
USP 797 requires Sterility testing for:
• Category 2 CSPs assigned a beyond-use date that requires sterility testing
• Category 3 CSPs
USP 797 requires a Bacterial Endotoxin Test (BET) for:
• Category 2 and Category 3 CSPs compounded from one or more nonsterile component(s)
• Multiple-dose CSPs
Sterility testing is a fundamental aspect of quality assurance for compounded pharmaceuticals, ensuring that samples tested are free from viable microorganisms. This is crucial for patient safety, as contaminated products can lead to serious health complications. Sterility testing is a control check meant to assess whether the compounding process has been performed in a manner that limits contamination, indicating the product is safe for use. It is also a critical component of many regulatory standards set forth by organizations like the FDA, USP, and State Boards of Pharmacy.
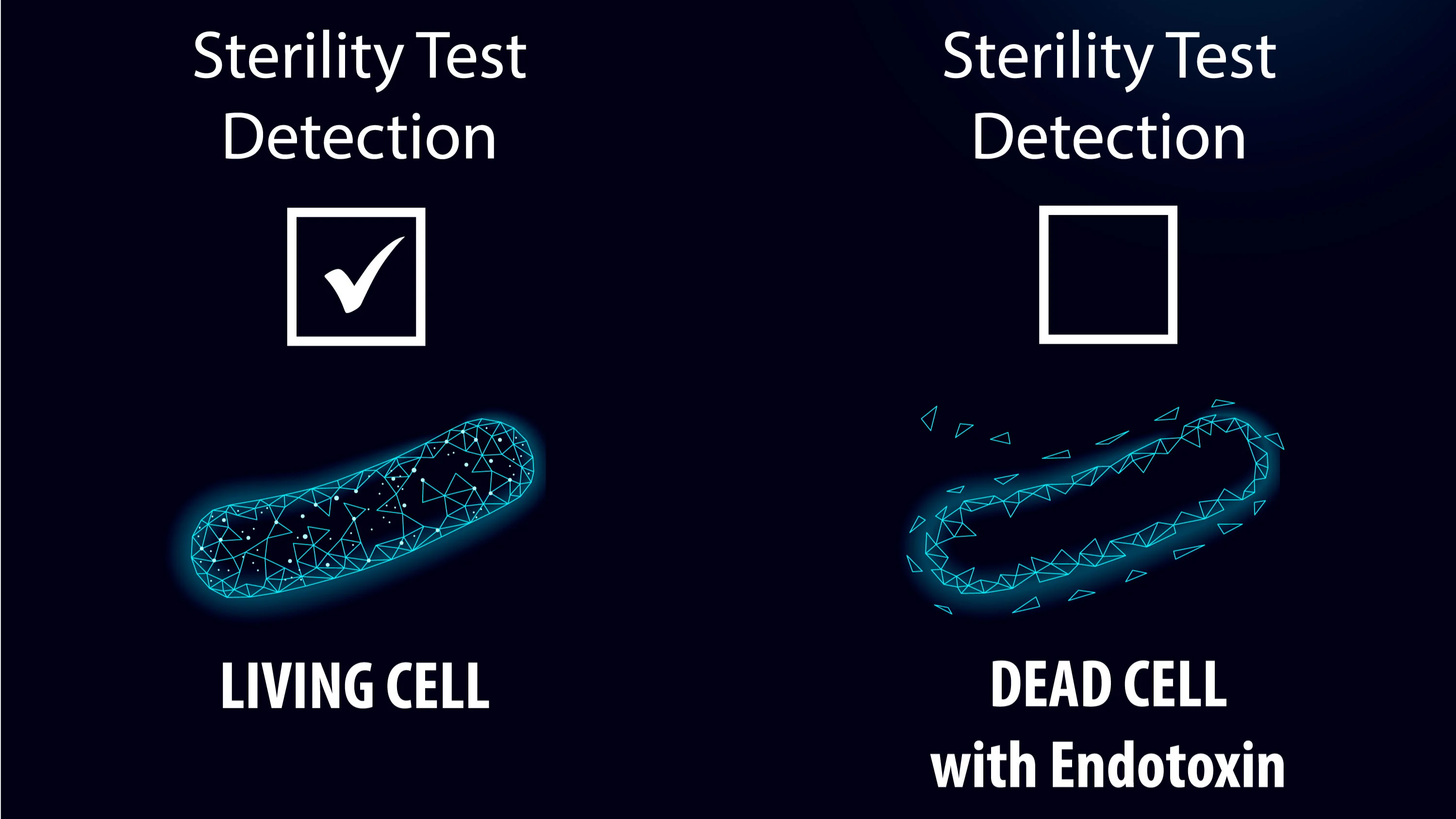
Importantly, a passing sterility test alone does not guarantee that a compounded product is completely safe. While sterility testing is meant to detect viable microorganisms, it is not designed to identify harmful bacterial byproducts, such as endotoxins. Endotoxins are toxic substances released from the outer membranes of certain bacteria, particularly Gram-negative bacteria, when they die or break apart. Even if a product is sterile and free from living bacteria, endotoxins may still be present and can result in dangerous immune responses in patients, including fever, inflammation, and septic shock. This is especially concerning when products are introduced into the bloodstream. A ‘Sterile’ test result does not ensure that a compounded product is free from these toxins. Given this, a robust testing regimen often includes both Sterility and Endotoxin testing, to provide assurance that a product meets patient safety expectations. By performing both Sterility and Endotoxin testing, a pharmacy is ensuring that their compounded medications undergo comprehensive safety assessments. This testing strategy provides ARL’s clients with confidence that the products are safe from both microbial contamination and the toxic effects of endotoxins, thus maintaining the highest standards of product quality and patient care.
For more information on sterility and endotoxin testing, contact ARL at 800-393-1595 or info@arlok.com.