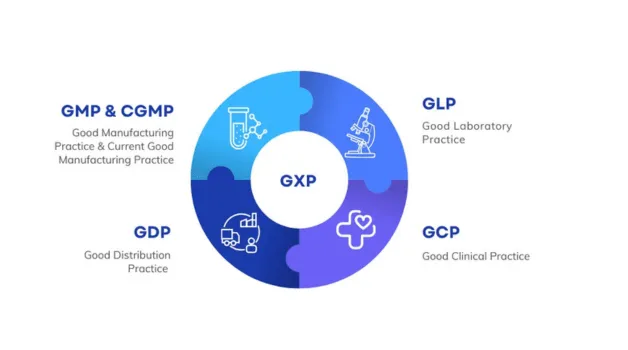
What is GxP Compliance?
GxP compliance is critical in industries where product quality, patient safety, and data integrity are non-negotiable. GxP stands for Good “x” Practices, where “x” refers to specific areas like manufacturing, distribution, laboratory, and clinical processes. Together, these standards ensure that organizations follow validated procedures, maintain proper documentation, and produce reliable, reproducible results.
Industries served by Rees Scientific, such as pharmaceuticals, biotechnology, blood and tissue banks, hospitals, research institutions, and CROs, rely heavily on GxP processes to meet regulatory requirements from the FDA, EMA, WHO, and other global agencies. Read More >