Identifying and Prioritizing Needs
USP <797>
- Review the existing Quality Program for gaps
- Revise the program with critical requirements from <797>
- Cleaning
- Environmental Quality and Control
- Facilities and Engineering Controls
- Handling, Labeling, Mixing, Release and BUD of Non-HD CSPs
- Personnel and Material Work flows Personnel Training and Assessment
- Quality Assurance: Policies, Procedures, Documentation and Records
USP <800>
- In addition to <797> requirements, revise the program with critical requirements from <800>
- Chemo wipe sampling
- Facilities and Engineering: Containment and Ventilation
- HD List and Risk Assessment
- Hazard Communication Program and Medical Surveillance
- Storage, Handling, Labeling, Mixing, Release and BUD of HD CSPs
What to do Once a QA Program is in Place
- Identify compliance priorities and resources available
- Determine which tasks should be outsourced
- Chemo wipe sampling
- Monthly environmental testing execution and support
- Personnel training and assessments: fingertip and media fill
- Policy and Procedure review and revision
- Review and approval of trend data and supporting documentation
Keys to Choosing a Vendor
- Assess their comprehension of the guidelines
- Check references
- Ensure vendor management is qualified
- Evaluate vendor staff and resumés
- Identify how out of specification data is reporting
- Review sample documentation
- Understand scheduling process and timelines for reports
Key Questions to Evaluating In-House vs. Outsourced
- Can we implement changes per current <797> and <800>?
- Can we maintain/calibrate necessary equipment- Incubators, Air Samplers, etc?
- Do we have dedicated and trained staff to develop, execute and manage our compliance efforts?
- Will our Quality Program and Documentation stand up to Board and JCAHO inspections?
- What is the risk and cost involved with In-House Staff performing key Quality, testing and lab tasks?
Quality Assurance Program
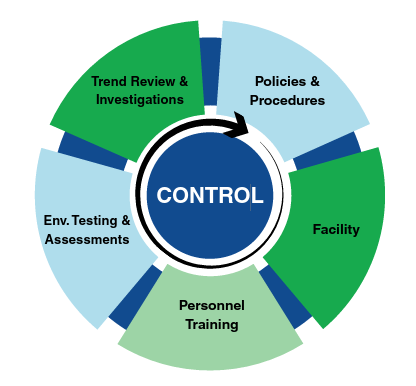
How Quality Solutions Can Help
- Certification & Environmental Testing
- Environmental Monitoring (EM) Kits
- Facility Design and Assessment
- Personnel Training & Assessment Support
- Quality System and Compliance Consulting
EM Kits
- Provide Kits and Reports to self perform routine environmental sampling
- Support your in-house testing with incubation and enumeration of test samples
Ensuring a Return on the Cost of Quality
800.935.1146
www.qualsolve.net
info@qualsolve.net





