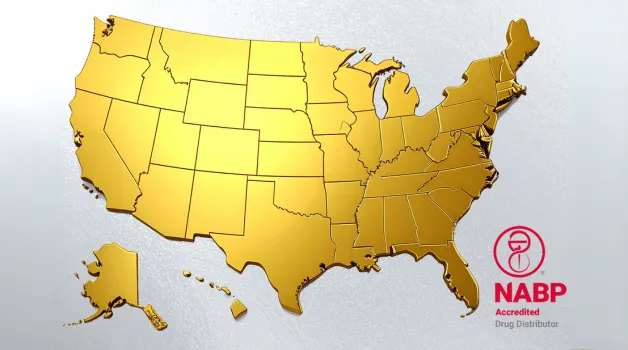
We’ve Reached Another Milestone!
Pharma Source Direct is proud to announce that we are officially authorized to sell in all 50 of the United States!
Nebraska? No doubt.
Florida? For sure.
Texas? Totally.
Minnesota? You betcha.
Indiana? Indubitably.
Pennsylvania? Positively.
Alaska? Absolutely.
Delaware? Definitely.
That’s right, ALL 50 States (and Puerto Rico)!
And, did we mention that Pharma Source Direct, Inc is also a proud recipient of the National Association of Boards of Pharmacy® (NABP®) Drug Distributor Accreditation?
As your trusted partner in business, Pharma Source handles FDA, DEA, and state compliance so you can focus on the most important things – running your business and taking care of your patients!
866-404-0846
CONTACT US

Last Wednesday, August 6th, FDA sent a letter to manufacturers, importers, and distributors of Animal-Derived Thyroid Products stating their intent to take action against unapproved ADT/DTE products, including bulk raw API used in pharmacy compounding.
Please note, FDA acknowledges the significant number of patients currently taking unapproved ADT/DTE products, and stated it will observe a 12-month enforcement grace period to give patients time to transition to FDA-approved treatments.
Of course, our friends at The Alliance for Pharmacy Compounding are keeping a close eye on this. Make sure you are supporting the advocacy group fighting to protect your compounding practice and patient access to the critical medicines they need!